
Security tightens at the JP Morgan Healthcare Conference in the aftermath of the murder of UnitedHealthcare CEO, Brian Thompson.
The 2025 J.P. Morgan Healthcare Conference (JPM) felt ... different this year.
As someone who eagerly makes the yearly pilgrimage to this keystone event to get a sense of where the biotech and pharma industries might be headed, I couldn’t help but notice the mix of feelings that defined the experience. It was like an emotional roller coaster — filled with enthusiasm, dread, and everything in between — with a slight undercurrent of tension that no one seemed willing to fully acknowledge.

One of many security dogs deployed during JPM 2025.
This year, the industry was rocked by the aftermath of UnitedHealthcare CEO Brian Thompson’s tragic murder last December, which led 11 major health insurance companies to quietly withdraw from JPM last week. Heightened security, bomb-sniffing dogs, and a palpable sense of caution surrounded Union Square. Thankfully, the conference, itself, concluded without incident.
Adding to the complexity, the backdrop of Donald Trump’s return as the 47th president, rising geopolitical tensions with China, economic struggles, and the continued rapid rise of artificial intelligence (AI) left many speculating about what lies ahead for the healthcare sector. Despite the uncertainty, at least San Francisco’s weather offered a rare sunny reprieve during the conference.
If you missed JPM 2025, here’s my take on the major themes and trends that emerged from this unforgettable gathering.
Ok, it’s time to talk about Trump (and it’s a huge mixed bag to go through)
Let’s first address the giant elephant in the room: President Donald Trump. Understandably, many Americans are holding their breath and wondering what impact his administration will have on the broader biotech and healthcare industries. This was a recurring question that dominated panel discussions and my many one-on-one conversations at JPM. For the sake of focus, I’ll confine this analysis strictly to Trump’s influence on healthcare and leave broader political implications aside.
Heading into JPM, I expected a unanimous consensus of doom and gloom, in which concerns about vaccine hesitancy and skepticism toward science seemed like inevitable talking points. But was that actually the case?
Well, not entirely.
Surprisingly, the sentiment among conference attendees was largely neutral and, in some cases, even borderline favorable. Some industry leaders even suggested that there are reasons to believe that Trump may actually be a net positive for biotech and healthcare innovation, depending on how one looks at it.

Matthew Herper (STAT News), Seema Verma (former CMS director), and Scott Gottlieb (former FDA commissioner) predict what’s next for the Trump administration during a STAT News panel at JPM 2025.
Former FDA Commissioner Dr. Scott Gottlieb and ex-CMS Director Seema Verma, both of whom worked closely with Trump during his first term, highlighted Trump’s keen interest for scientific advancements. At a STAT News panel during JPM, Dr. Gottlieb cited Trump’s excitement over KYMRIAH, the first FDA-approved cell therapy for certain blood cancers, as one of many proof points of the president’s eagerness.
“The president has a lot of enthusiasm about the science,” Dr. Gottlieb remarked.
Verma echoed similar sentiments, adding, “What was misunderstood is that he [Trump] really believes people should have access to high-quality healthcare. It’s a deep concern of his.”
This optimism among many speakers at JPM sparked a call to action for the industry to proactively seek collaboration with the new administration. Because whether one likes it or not, Trump’s going to be our president for the next four years. As former CMS director under the Biden Administration, Meena Seshamani, noted during a separate STAT panel, “Moving forward, it’s about being able to find common ground, regardless of who or which party is in office.”
Perhaps not so surprising, a number of biotech executives and investors at JPM appeared particularly enthusiastic about the potential for business growth under President Trump. The anticipated regulatory changes, including the departure of Lina Khan as chair of the Federal Trade Commission (FTC) — who, historically, has been tough on biotech megamergers and famously blocking the $7.1 billion Illumina & Grail deal — could pave the way for more lenient merger and acquisition (M&A) policies. This is significant, as M&A deals are a vital source of capital and innovation for many biotech companies.
Mark McKenna, Mirador Therapeutics’ CEO, speaking at an Endpoints News panel, explained, “I also think that the changes at the FTC can open up new doors with respect to the size and complexity of deals getting done. We won’t go back to 2018, but filling gaps with external innovation can be found with bigger M&A synergies.”
Another hot topic at JPM was the initiative spearheaded by the so-called Department of Government Efficiency (DOGE), created by Elon Musk and biotech veteran Vivek Ramaswamy. (The name is somewhat misleading, as DOGE is not an official government entity). The DOGE team aims to recommend ways to streamline government bureaucracy and reduce inefficiencies, which could have ripple effects throughout the healthcare industry.
In response to DOGE, Robert Nelson, co-founder and managing director of Arch Venture Partners, a very prominent firm that has made successful investments in biotech startups, believes that such a “stirring” is necessary to clean house and reduce waste in a current system that isn’t benefitting patients as rapidly as it could.

Andrew Dunn (Endpoints) and Bob Nelson (Arch Venture Partners) discuss the state of biotech investing during an Endpoints News fireside chat at JPM 2025.
“I’m supporting anybody who wants to stir it up,” said Nelson, during another Endpoints panel. “What’s going to happen in the next 90 days is going to be bigger and more rapid than people think. It’ll be good for this industry. But you’ll have to hold your breath at the same time.” Nelson’s comment drew a bit of controversy from people I talked to after his fireside chat, with some being very supportive of his perspective, while others were left disturbed.
Despite the pockets of optimism around Trump, however, legitimate concerns persist.
The one unequivocally universal sentiment at JPM was the pushback against Trump’s appointment of Robert F. Kennedy Jr. (RFK Jr.) as secretary of the U.S. Department of Health and Human Services (HHS). Known for his history of vaccine skepticism, RFK Jr.’s new HHS role has sparked fears about potential disruptions to vaccine regulation and immunization schedules, particularly for children. Dr. Gottlieb, during the conference, warned that the country is already seeing vaccination rates fall as low as 80% in some states. He believes if it drops another 5%, we risk outbreaks of diseases, such as measles, which have long been kept under control.
“I don’t think he [RFK Jr.] should be [secretary of] HHS. I'm concerned with the positions he’s taken. They’re not contrarian views. They’re flawed views around vaccines,” Dr. Gottlieb said during JPM, who also famously provided his views on CNBC.
If history is any indication of what Trump might do this term, another concern is that his team will undo much of the progress made by previous presidential administrations, including key programs such as Medicare and former President Barack Obama’s Affordable Care Act (ACA).
“Medicare and ACA are huge targets for the incoming administration. We have to keep an eye on both programs because they are vulnerable to current cost-cutting discussions on Capitol Hill, which can affect many Americans,” warned Juliette Cubanski, deputy director of the Program on Medicare Policy at KFF, during an Endpoints News JPM panel focused on Trump’s impact on healthcare policy.
So what’s my take on all of this?
As I see it, there seems to be two competing visions within the Trump administration: one focused on fostering scientific innovation and data-driven progress, and another advocating for balancing the books and deregulation. How these two agendas will coexist remains uncertain.
One clear shift, however, was the notable, and surprising, change in sentiment toward Trump at JPM this year. Pharma and biotech stakeholders appear to be “all in” on collaborating with the administration — perhaps to avoid drawing Trump’s ire, or perhaps due to genuine optimism about the opportunities ahead.
With regards to RFK Jr., the true litmus test on where Trump/RFK Jr. lands on vaccines will be the upcoming Advisory Committee on Immunization Practices (ACIP) meeting that will take place towards the end of February. Only then will we get a decent pulse on what will happen at the FDA and CDC levels. Until the ACIP meeting, it’s all speculation.
For now, it’s evident that the next four years will bring challenges and opportunities for the biotech and healthcare industries. Regardless of one’s personal politics, the industry’s broader mission of helping patients must remain the top priority. Collaboration with the new administration, however complex, will be critical to achieving this goal.
M&A Surges Ahead to Counter Patent Cliffs; IPOs Remain Unfavorable
The JPM conference kicked off with a bang, marked by three high-profile mergers and acquisitions (M&A) deals that reignited activity in the biopharma sector after a period of relative stagnation compared to previous years.
The marquee announcement was Johnson & Johnson’s $14.6 billion acquisition of Intra-Cellular Therapies. This move adds CAPLYTA — a treatment for bipolar I and II disorders, depression, and schizophrenia — to J&J’s portfolio, while bolstering its growing neuroscience pipeline. This transaction is the largest pharma M&A deal since 2023, surpassing last year’s headline deal, in which Vertex acquired Alpine Immune Sciences for $4.9 billion.
Not all deals, however, involved marketed drugs, signaling a potential “light” return to investing in early stage assets. Eli Lilly’s $2.5 billion acquisition of Scorpion Therapeutics focused on a PI3Kα inhibitor program for breast cancer, currently in Phase 1/2 trials. GSK also joined the fray with a $1 billion buyout of IDRx, acquiring its Phase 1 asset targeting gastrointestinal stromal tumors (GIST), a rare form of cancer.
There is a noticeable wave of optimism surrounding M&A this year. Industry leaders at JPM expressed a bullish outlook, anticipating a sustained surge in deal-making through 2025 and beyond.
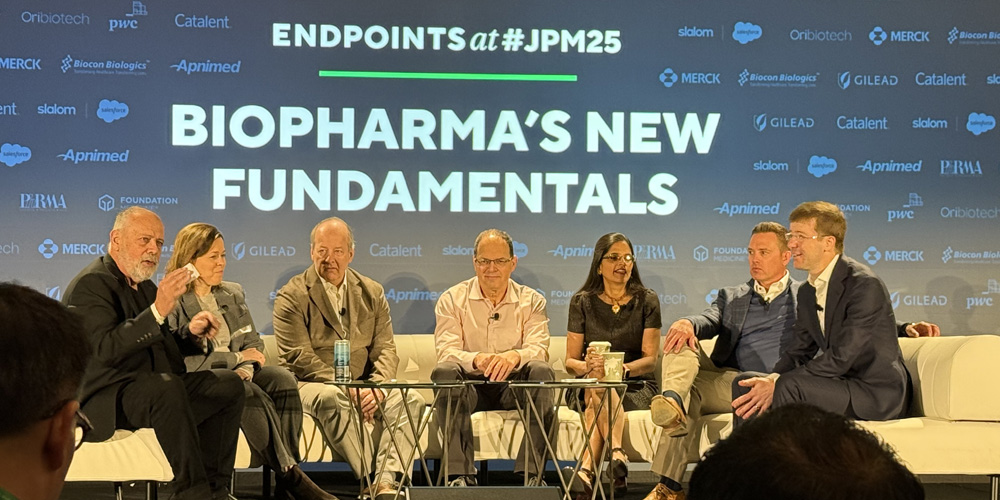
John Carroll (Endpoints), Julie Rozenblyum (BMS), Sander Slootweg (Forbion), Aharon Gal (Novartis), Aradhana Sarin (AstraZeneca), Marck McKenna (Mirador Therapeutics), and Roel van den Akker (PwC US) discuss the future of dealmaking in 2025 during an Endpoints News JPM panel.
Roel van den Akker, principal at PwC, shared during an Endpoints News panel: “Going forward, we’re very optimistic for increased levels of [M&A] activity.”
Similarly, Eric Tokat of Centerview Partners highlighted a growing interest in early stage assets, noting: “The profile of the deals today signals that future deal flow will likely mirror these trends. I believe this year will showcase a resurgence in larger, commercial-stage transactions, while maintaining momentum in early stage pipeline acquisitions.”
The resurgence we’re seeing in M&A activity can be summed up in two words: Patent cliffs.
While recent acquisitions provide a welcome boost to the pharma sector, the looming expiration of blockbuster drug patents threatens to erode billions in revenue for major pharma players.
Take, for example, Johnson & Johnson’s DARZALEX, a leading cancer drug developed by Genmab, whose key patents expire in the late 2020s. Meanwhile, Merck faces a monumental challenge with KEYTRUDA — its $25 billion cancer blockbuster, accounting for nearly 40% of the company’s global sales — set to go generic by 2028. Merck also anticipates patent expirations for JANUVIA (type 2 diabetes) in 2026 and GARDASIL (HPV vaccine) in 2028.
These looming cliffs are forcing companies to act decisively by pursuing acquisitions to mitigate revenue gaps and diversify their pipelines.
But what about the outlook for initial public offerings (IPOs) in biotech at JPM?
Despite a gradual market recovery, biotech IPOs remain ill-advised by experts at JPM. Investors continue to encourage companies to build resilience through M&A rather than relying on the volatile public markets. According to Sander Slootweg, managing partner at venture capital (VC) firm Forbion, approximately 80% of biotech exits have occurred through M&A rather than IPOs.
“From a VC perspective, we see a trend toward making companies less reliant on IPOs. We prefer to bank on M&A as a private company, while treating IPOs as optional,” Slootweg said during an Endpoints News panel session at JPM.
Mirador Therapeutics’ CEO, Mark McKenna echoed this sentiment: “Capitalize the company so you’re not dependent on public markets. You don’t want to get in the public market and then get punched in the face.”
From my perspective, although the early momentum at JPM suggests a promising year for M&A, tempered optimism is warranted (the XBI index, which tracks the performance of U.S. biotechnology stocks, remained rather stagnant throughout JPM). And we’ve seen this similar narrative unfold at last year’s conference, with predictions of a transformative 2024 following notable, but smaller, M&A announcements.
This year’s stronger start suggests an uptick in activity and potential megadeals. However, navigating market uncertainties and geopolitical shifts with China, which I’ll be exploring next, may hinder the scale of the anticipated resurgence, at least in the short term.
The Rise of China’s Biotech Industry, and Its Impact on the U.S. Biotech Ecosystem
For virtually all JPM attendees, the rapid ascent of China’s biotech sector has become impossible to ignore. It’s creating significant competitive pressure on U.S. companies, compounded by the backdrop of rising geopolitical tensions between the two nations. To put it simply, China’s ability to execute drug discovery and development more quickly and at a fraction of the cost is fueling an explosion of assets emerging from the country.
As John Carroll, editor-in-chief of Endpoints News, noted during a JPM panel session, the industry’s new mantra is simple: “Speed is currency.”
Dr. Scott Gottlieb echoed this sentiment, pointing out a major shift in global investor and scientific focus. Today, nearly 50% of investigational new drug (IND) work in oncology originates from China, forcing investors and companies to rethink their strategies for licensing and investment. Houman Ashrafian, Sanofi’s EVP and head of Research & Development, suggested during an Endpoints News fireside chat at JPM that the traditional VC model may evolve as investors increasingly embrace emerging and established markets.
At JPM, these dynamics have sparked important conversations about how U.S. biotech and pharma companies can respond. Discussions have centered on reducing R&D costs to remain competitive, while simultaneously pushing the boundaries of innovation by developing groundbreaking drugs based on novel biology.
Despite the challenges, there’s widespread agreement that U.S. companies continue to lead the global biotech sector through innovation. Aradhana Sarin, AstraZeneca’s CFO and executive director, emphasized this point:
“Innovation in the U.S. is still the best in the world. If you look at new patents filed, the U.S. remains at an all-time high, followed by China. However, there’s significant innovation emerging from other parts of the globe.”
Eric Tokat, speaking during an Endpoints News panel at JPM, offered a similar perspective:
“Good science is happening in China, and the costs there are very low. But the U.S. remains the core epicenter of innovation. It will be interesting to see how the new [Trump] administration approaches these challenges.”
The rise of China’s biotech industry is undoubtedly reshaping the global landscape. For U.S. companies, staying competitive means finding ways to lower costs without sacrificing quality and continuing to lead with innovation. At the same time, investors and companies will need to adapt to a more globalized and competitive biotech ecosystem, one in which emerging markets, such as China, play an increasingly central role.
AI Continues to Dominate JPM Discussions, with a Tonal Shift Towards Operations and “Digital FTEs”
Artificial Intelligence (AI) was the darling of conversations at last year’s JPM conference, with discussions centered on its potential to revolutionize drug discovery and target previously intractable diseases. This year, the discourse evolved. While the excitement around AI’s promise remains strong, a notable shift in focus emerged, which more strongly emphasized leveraging AI to enhance operational efficiency in biotech and pharma.
On Day one of JPM, NVIDIA, one of the world leaders in AI computing, reaffirmed its commitment to the healthcare and life sciences industries by announcing partnerships with IQVIA, Illumina, Mayo Clinic, and the Arc Institute. These collaborations aim to develop AI-driven solutions that advance human health. However, despite these advancements, industry leaders had a more pragmatic outlook on AI’s immediate potential this year.
The tone at JPM reflected more restrained expectations for AI in drug discovery. While AI holds immense promise for developing novel therapies, experts acknowledge that the technology is not yet ready to deliver fully AI-designed medicines in the near term. As Marc Tessier-Lavigne, CEO of Xaira Therapeutics, noted during an Endpoints News fireside chat, “While it’s good to be aspirational with AI, it’s equally important to remain practical about its current limitations.”
Instead, the spotlight turned to AI’s ability to streamline company operations and reduce inefficiencies. Siddhartha Bhattacharya, PwC’s principal for Pharmaceutical & Life Sciences, highlighted the value of “digital full-time equivalents (FTEs),” which essentially are AI agents acting as virtual work companions to augment human workers.

Arsalan Arif (Endpoints News), Patrick Loerch (Gilead), and Siddhartha Bhattacharya (PwC) debate the future of AI in healthcare during an Endpoints News panel at JPM.
Digital FTEs are already transforming workflows in biotech and pharma. Tasks that once took hours are now being completed in minutes. For instance, a medical writing project that traditionally required 20 hours of work can now be completed in under two hours, including human oversight to edit and refine the final deliverable.
According to Bhattacharya, these AI agents will evolve over time. Initially, they automate small tasks. But they will gradually handle groups of tasks and eventually full roles. “It’s something that people can’t ignore,” Bhattacharya remarked during an Endpoints News panel at JPM.
Not everyone agrees on the extent of AI’s capabilities. Patrick Loerch, Gilead’s SVP of Clinical Data Science, argued that while AI can enhance productivity, its current competency is equivalent to that of a college graduate. In regulated environments, such as healthcare, careful integration and human oversight are crucial to ensure quality and mitigate risks, such as data drift and bias.
“I don’t believe large language models [LLMs] and AI will replace employees, but I do think it’ll help enhance their jobs,” Loerch countered during the same panel with Bhattacharya. “It’s phenomenal what AI can do, but in healthcare, we need to think carefully about workflows and where to insert the appropriate human oversight.”
From the perspective of scientists who perform bench research, AI is seen as an empowering tool rather than a replacement. AI excels at generating hypotheses, but relies on human expertise for testing and validation, which requires paying close attention to the input and the output of an AI system.
David Fajgenbaum, associate professor of Medicine at Penn Medicine, emphasized during a panel at the “Innovation @ Penn” JPM event that human involvement remains critical. “You have to be very careful about the outputs. That’s why it’s important to keep humans in the loop, for now.”

JPM attendees gather en masse to learn from AI experts at Wharton San Francisco’s “Innovation @ Penn: Real World Implementation of AI and Healthcare” panel.
Fajgenbaum also highlighted AI’s success in drug repurposing, in which algorithms identify new uses for existing drugs. This approach has already saved lives and demonstrated AI’s ability to deliver a near-term impact in healthcare.
As an observer of the AI conversation at JPM, the discussions revealed two opposing forces for AI’s future in biotech and healthcare. On one side, leaders see AI as a tool to enhance human work and address inefficiencies. On the other, some envision a future in which AI could fully replace human roles, although this view remains largely unspoken.
On a totally separate note outside the JPM conference, it appears that some groups in the broader tech industry on the West Coast are leaning towards the latter vision. A number of billboards in San Francisco promoting AI’s capabilities suggests a strong push to automate jobs entirely. This trend raises questions about the ethical and societal implications of such advancements.

Pro-digital FTE billboard spotted on the US-101 highway in South San Francisco.
While AI continues to dominate discussions, the healthcare industry remains at a crossroads. Whether AI becomes a partner to human workers or a replacement will depend on how companies balance innovation with responsibility. For now, the focus remains on leveraging AI’s strengths to empower people, enhance efficiency, and unlock new possibilities in biotech and beyond.
Closing thoughts
What a whirlwind of a week at JPM 2025. While the world, and our industry, feels more uncertain than ever, there were moments of clarity and hope that left me optimistic for the year ahead.
From hearing Nobel Laureate Jennifer Doudna share her inspiring vision for the future of CRISPR gene editing...

Nobel laureate, Jennifer Doudna explains her vision for the future of CRISPR.
… to making new friends with like-minded peers who care deeply about the industry, there were highlights that reminded me why JPM is such a unique and special event.
Despite the challenges looming on the horizon, there’s still so much to look forward to in biotech this year. Stay tuned as we at HDMZ continue to share insights on how this exciting, unpredictable journey unfolds.
Note: All quotes have been edited for brevity and clarity.

About the author
John Kang is the Senior Vice President of Public Relations at HDMZ, leveraging his extensive expertise in communications and the life sciences to lead corporate communications strategies for prominent biotech and life science organizations across domestic and international markets.


